Product Data Sheet
Safety Data Sheet
Quinidine Base (CAS-No.: 56-54-2), a stereoisomer of the well-known Cinchona Alkaloid compound Quinine, contains the framework of sophisticated highly efficient organocatalysts. Quinidine Base and its derivatives – for example Cupreidine – enables the synthesis of complex chiral compounds in high yield and with remarkable high enantioselectivitity. Please review the Chiral Screening Data Base containing hundreds of examples of asymmetric reactions using cinchona alkaloids. Compared to Quinine, the opposite configuration of target compounds can be obtained. The catalysts, are eco-friendly, highly stable and available for reasonable prices. As green catalyst these compounds are widely used in large-scale applications. Apart from its application as chiral organocatalyst Quinidine is used as a racemic resolution agent in industrial racemic resolution processes.
Pharmaceutical Application
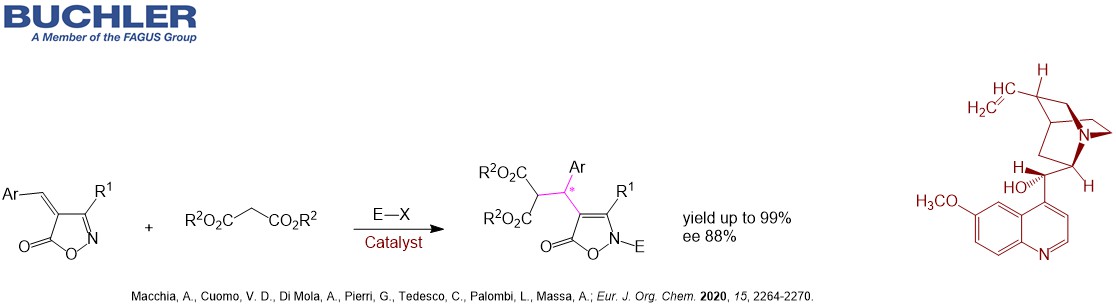
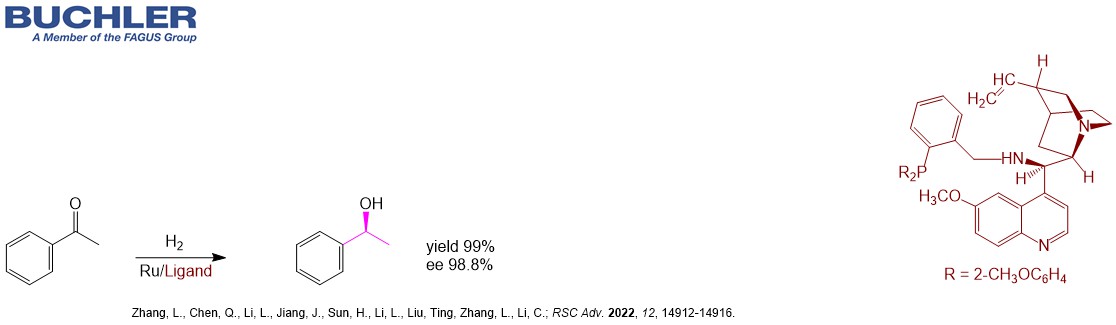
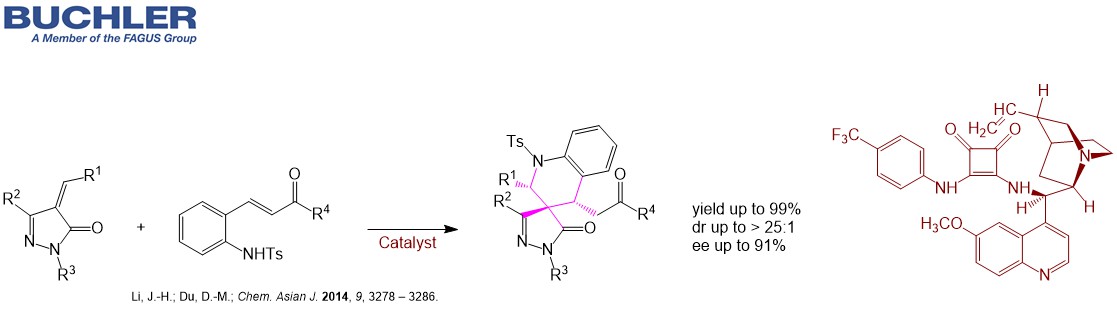
The synthesis of the API Droxidopa is accomplished by Quinidine separating the enantiomers. The different Cinchona Alkaloids are often used in desymmetrization processes in order to obtain versatile chiral building blocks. For instance Quinidine promotes the ring opening of prochiral cyclic anhydrides in a highly efficient manner with a remarkable enantioselectivity up to 99 % ee. Desymmetrization is the key step in the synthesis of the API Telaprevir. Apart from the asymmetric application Quinidine as a natural alkaloid has been used for decades to treat heart conditions such as arrhythmia. In combination with Dextromethorphan it treats the Pseudobulbar affect (PBA) – a condition that is characterized by episodes of sudden uncontrollable and inappropriate laughing or crying. Buchler is a reliable supplier of Quinidine Salts such as Hydrocloride, Sulfate or Gluconate.
Physical properties
Quinidine Base (CAS-No.: 56-54-2) is a white or almost white, crystalline powder, being very soluble in ethanol and chloroform . The melting point is in the range of 168-173.4 °C. This compound is not hygroscopic and contains less than 1.0 % water. The compound could be stored at ambient conditions in well-closed, light-resistant containers.
Synonyms
Quinidine anhydrous, Chinidin, (+)-(8β,9S)-6′-Methoxycinchonan-9-ol, Conquinine, 6`-Methoxycinchonine, (8R,9S)-6`-Methoxycinchonan-9-ol, (8β,9S)-6`-Methoxycinchonan-9-ol and (S)-[(2R,4S,5R)-5-Ethenyl-1-azabicyclo[2.2.2]oct-2-yl](6-methoxyquinolin-4-yl)methanol
NMR-data
The following spectroscopic techniques were used to provide structural evidence for Quinidine Base by NMR technology: 1H NMR, 13C NMR, 1H-1H COSY, 1H-13C HSQC, 13C-1H HMBC, 1H-1H NOESY, 15N-1H HMBC.
NMR spectra of the structural elucidation can be viewed here:
NMR – Quinidine BaseAssociated Reactions
175 reactions found.
But only 10 are listed.
Please login below to see more.