Product Data Sheet
Safety Data Sheet
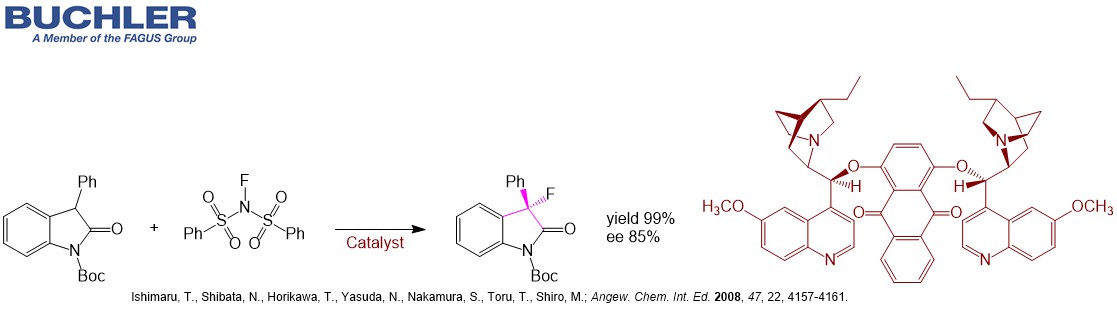
Dihydroquinidine Base – DHQD (CAS-No. 1435-55-8) is a cinchona alkaloid closely related to quinidine. Compared to Quinidine Base the double bond is hydrogenated, but the stereochemistry is the same. DHQD is widely used as a chiral ligand / chiral catalyst in asymmetric synthesis. Well-known derivatives of Dihydroquinidine like (DHQD)2PHAL, (DHQD)2AQN or (DHQD)2PYR enable highly enantioselective reactions.
Please also take a look into our free of charge Chiral Catalyst Search Database, containing a variety of examples.
Application
Typical applications of the chiral Cinchona alkaloid dimer catalysts based on Dihydroquinidine (DHQD) are summarized as follows:
- (DHQD)2PHAL: allylation, amination, bromohydroxylation, bromoesterfication, bromination, chlorination, dichlorination, dihydroxylation, dithiotrifluoromethylation, fluorination, halolactonization, haloetherfications, kinetic resolution, lactonization, Mannich reaction, Michael, sulfenylation and trifluoromethylation
- (DHQD)2AQN: aldol, allylic alkylation, allylic amination, amination, desymmetrization, fluorination, fluoromethylation, kinetic resolution of biaryls and Michael addition
- (DHQD)2PYR: fluorination, Michael addition, vinylogous Michael addition, semipinacol rearrangement, sulfenylations and trifuoromethylthiolation
- (DHQD)2CLB: aminohydroxylation
Physical properties
Dihydroquinidine Base (CAS-No.: 1435-55-8) is a white or almost white, crystalline powder which is soluble in ethanol. The melting point is 168.5 °C. This compound is not hygroscopic and contains less than1.0 % water. Dihydroquinidine could be stored at ambient conditions in well-closed, light-resistant containers.
Synonyms
DHQD, Hydroquinidine, (8R,9S)-10,11-Dihydro-6`-methoxycinchonan-9-ol, (8β,9S)-10,11-Dihydro-6`-methoxycinchonan-9-ol and (S)-[(2R,4S,5R)-5-Ethyl-1-azabicyclo[2.2.2]oct-2-yl](6-methoxyquinolin-4-yl)methanol.
NMR-data
The following spectroscopic techniques were used to provide structural evidence for Dihydroquinidine Base by NMR technology: 1H NMR, 13C NMR, 1H-1H COSY, 1H-13C HSQC, 13C-1H HMBC, 1H-1H NOESY, 15N-1H HMBC.
NMR spectra of the structural elucidation can be viewed here:
NMR – Dihydroquinidine BaseAssociated Reactions
82 reactions found.
But only 10 are listed.
Please login below to see more.