Product Data Sheet
Safety Data Sheet
Introduction
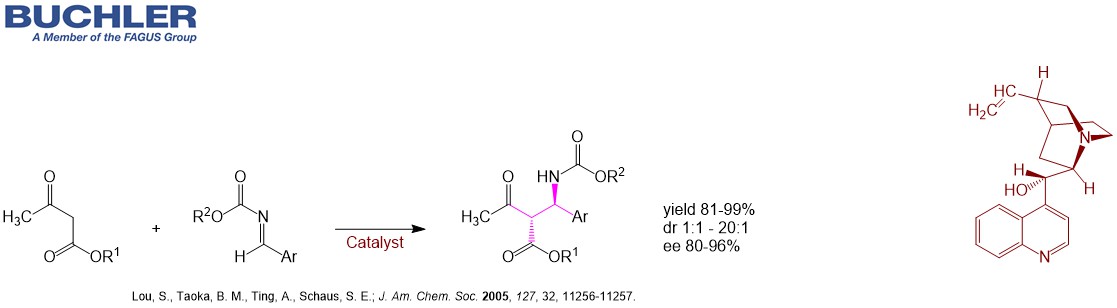
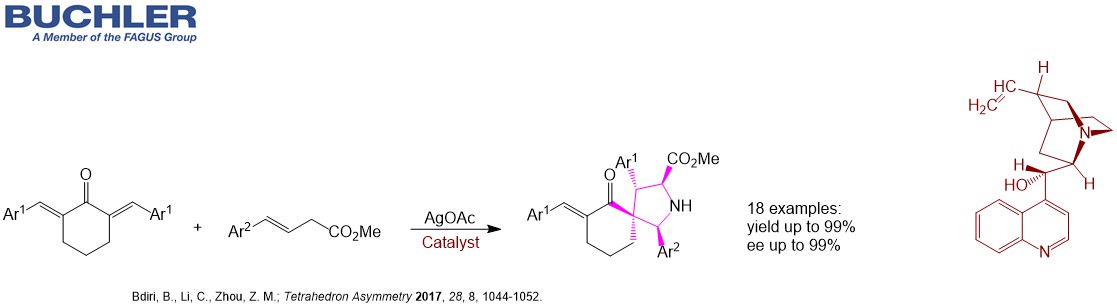
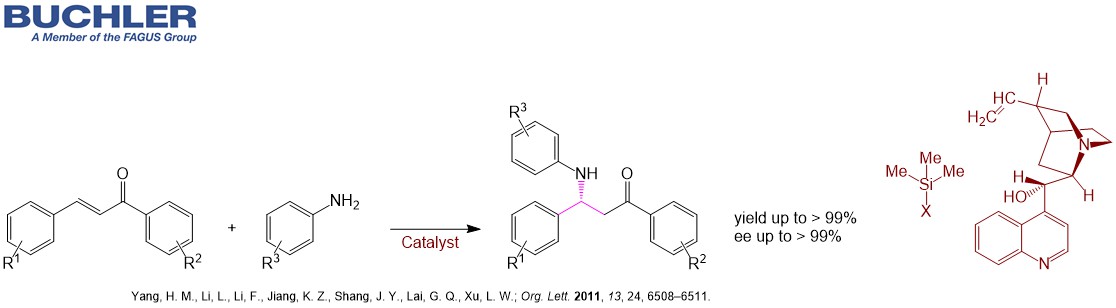
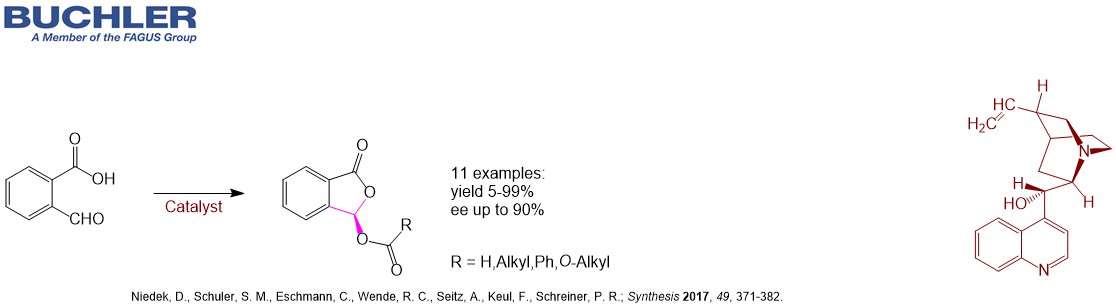
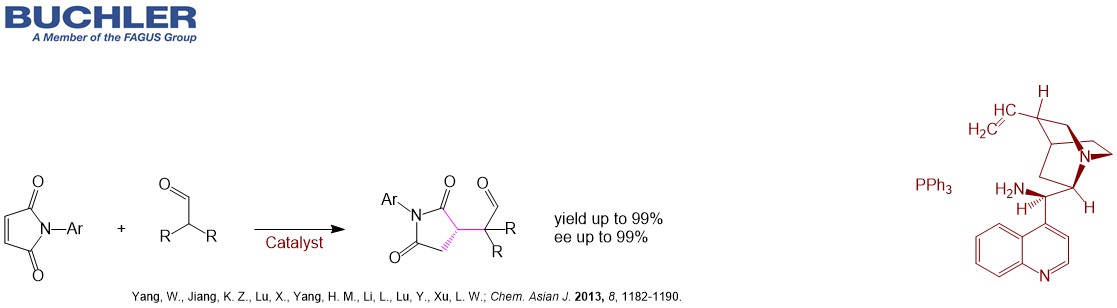
Cinchonine Base (CAS-No. 118-10-5), a pseudoenantiomer of Cinchonidine, is a well-known chiral compound widely used as a racemic resolution agent since decades. Apart from this application Cinchonine Base and its Cinchona alkaloid derivatives acts as an efficient enantioselective organocatalyst in a wide variety of asymmetric reactions. Multiple examples can be reviewed in our free of charge chiral catalyst search Database. Nowadays, the term asymmetric oganocatalysis covers a wide range of organic processes and methodologies, providing efficient and environmentally friendly access to enantiomerically pure compounds. Cinchonine is a versatile tool in applied Green Chemistry.
Find other examples of Chiral Organocatalysts in the Buchler Chiral Catalyst Search Database.
Physical properties
Cinchonine (CAS-No.: 118-10-5) is a white or almost white, crystalline powder or it consists of fine, colourless needles, being soluble in ethanol and methanol. In contrast to the well-known cinchona alkaloids, the solubility of Cinchonine in other solvnets is limited. The melting point is in the range of 250-255°C. This compound is not hygroscopic and contains less than 1.0 % water. According to the literatur the pka values have been determined: pk1 = 5.85 and pk2 = 9.92. Cinchonine could be stored at ambient conditions in well-closed, light-resistant containers.
Synonyms
(8R,9S)-Cinchonan-9-ol, (8β,9S)-Cinchonan-9-ol and (S)-[(2R,4S,5R)-5-Ethenyl-1-azabicyclo[2.2.2]oct-2-yl](quinolin-4-yl)methanol.
NMR-data
The following spectroscopic techniques were used to provide structural evidence for Cinchonine Base by NMR technology: 1H NMR, 13C NMR, 1H-1H COSY, 1H-13C HSQC, 13C-1H HMBC, 1H-1H NOESY, 15N-1H HMBC.
NMR spectra of the structural elucidation can be viewed here:
NMR – Cinchonine BaseAssociated Reactions
172 reactions found.
But only 10 are listed.
Please login below to see more.