Asymmetric oxohydroxylation of α-alkyl enoates with potassium permanganate catalyzed by monocationic quaternary...
Buchler Glossary
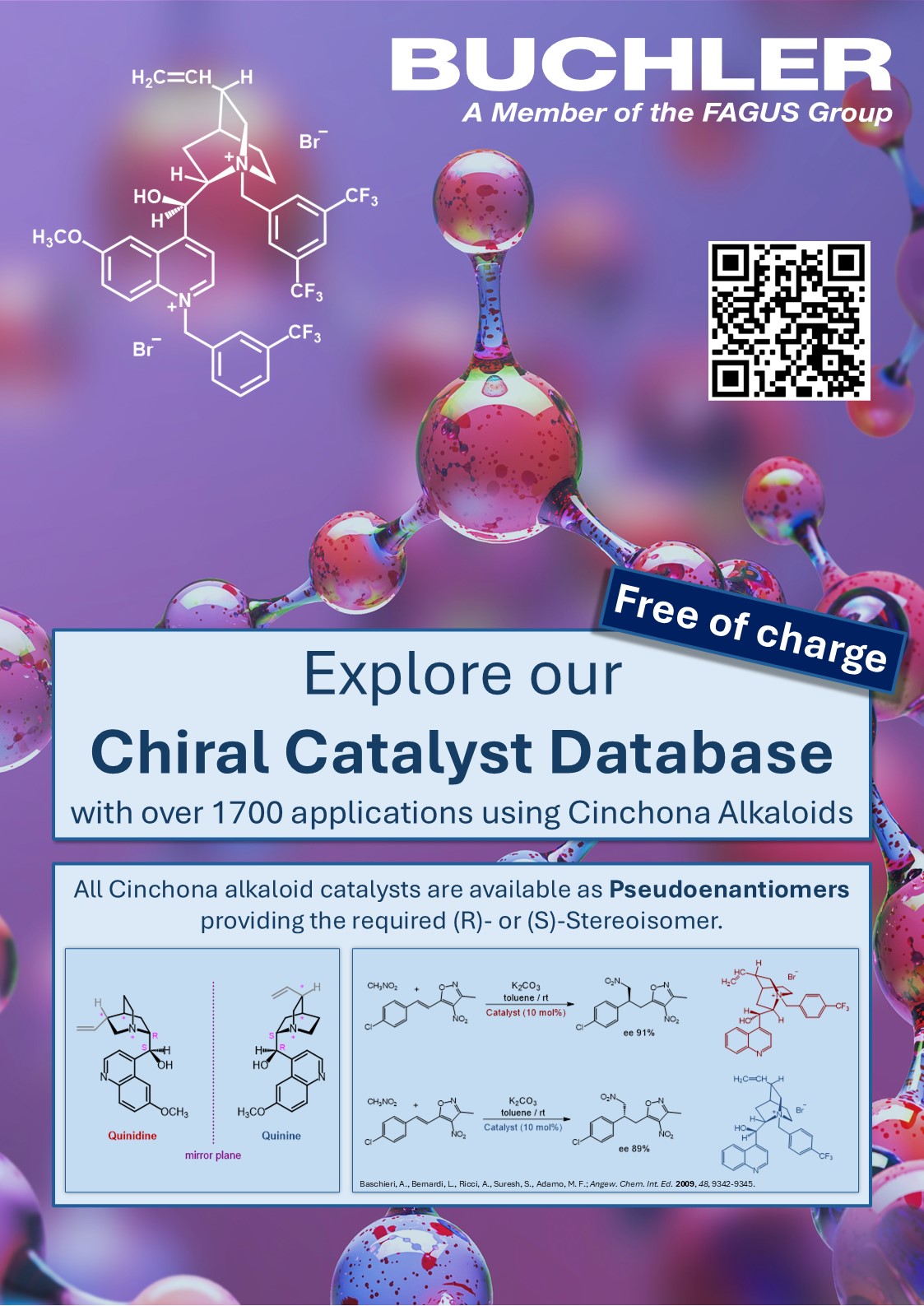
Organocatalysts are one of the bravest attempts to mimic enzyme catalysis that Mother Nature uses. A Chiral Organocatalyst is a small organic molecule that can catalyze reactions in the absence of metals or metal ions. Cinchona Alkaloids are chiral organic compounds that can be used as chiral catalyst in many reactions such as aldol condensation, Mannich reaction, Henry reaction or Michael addition.
To be able to get an overview of Cinchona Alkaloids as Organocatalyst please find our table of products here: Overview on Cinchona Alkaloids as Catalysts.
Application
Hundreds of different reaction examples using Cinchona alkaloids as chiral organocatalyst can be found in our Chiral Catalyst Search.
Further Articles:
(DHQ)2PHAL (CAS-No. 140924-50-1)
(DHQ)2PHAL - Dihydroquinine 1,4-phthalazinediyl diether (CAS-No. 140924-50-1) The well-known Cinchona alkaloid...
(DHQD)2PHAL (CAS-No. 140853-10-7)
(DHQD)2PHAL - Dihydroquinidine 1,4-phthalazinediyl diether (CAS-No. 140853-10-7) The well-known Cinchona alkaloid...
1,2-Addition
1,2-Addition is a type of organic chemical reaction that involves the addition of functional groups to the 1st and 2nd...
1,4-Addition (conjugate addition)
The Michael reaction is the conjugate 1,4-Addition of a resonance stabilized carbanion (michael donor) to an activated...
6`-Aminocinchonine (CAS-No. 2143936-31-4)
6´-Aminocinchonine Base (CAS-No. 2143936-31-4), a pseudoenantiomer of 6´-Aminocinchonidine, has the same...