Enantioselective Organocatalysts
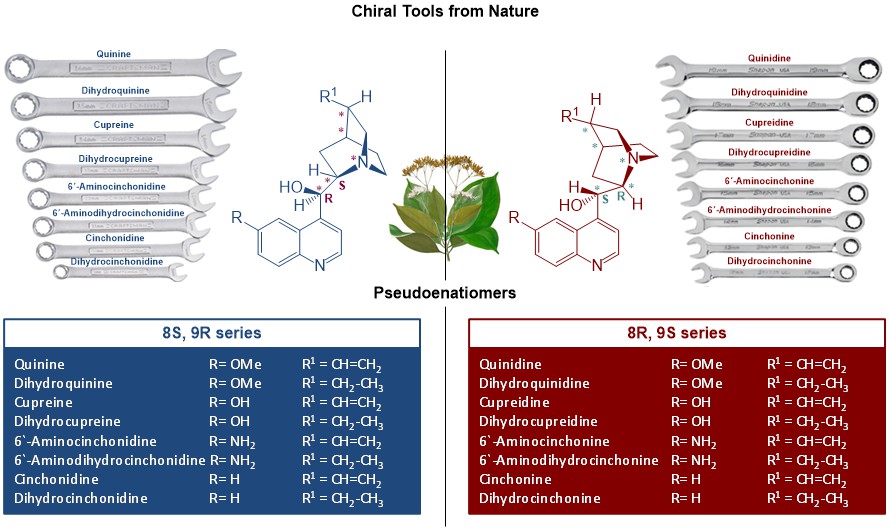
Cinchona alkaloids are recognized as a privileged class of compounds being widely used as chiral organocatalysts/ligands/phase transfer catalysts in asymmetric synthesis and in racemic resolution processes. One of the most interesting features of this well-known family of alkaloids is their availability in two pseudo-enantiomeric forms (BLUE SERIES and RED SERIES). Cinchona catalysts can therefore provide access to both enantiomers of a required product.
Key Features of Cinchona Alkaloids:
- serve as highly versatile catalysts for a broad spectrum of enantioselective transformations
- are highly enantioselective catalysts for more than 100 types of asymmetric reactions
- form 2 pairs of pseudoenantiomers
- enables a target oriented production of the required (R) or (S) stereoisomer
- available in multi ton scale
- are usually robust and tolerate different reaction conditions
- soluble in a wide range of organic solvents and aqueous solutions
- recyclable and therefore cost efficient
- can be immobilized via vinyl side chain
Search for enantioselective reactions with Cinchona alkaloids. More than 100 examples for different reaction types are available: