Asymmetric oxohydroxylation of α-alkyl enoates with potassium permanganate catalyzed by monocationic quaternary...
Buchler Glossary
Michael addition / Enantioselective Michael Addition is one of the most frequently used C-C bond-forming reactions in organic synthesis. By the way it is certainly one of the most powerful. The Michael addition is a simple reaction between nucleophiles and activated olefins and alkynes. The nucleophile adds across a carbon–carbon multiple bond. Michael additions are in general C-C bond formation reactions in which nucleophilic addition of a carbanion or another nucleophile to an α,β-unsaturated carbonyl compound containing an electron withdrawing group occurs.
The Enantioselective Michael Addition can be carried out in particular using catalysts based on cinchona alkaloids with high enantiomeric excesses. Following the principal of green chemistry, mild reaction condition are applied. The catalyst are eco-friendly, non-toxic, highly stable and available for reasonable prices. Therefore they are widely used in large-scale applications. Furthermore the catalyst can be easily recycled.
Further types of Michael Addition
Over the years, Michael additions (Reaction category according to RXNO Ontology: RXNO_0000009) in many versions known as aza-Michael, thio (sulfa)-Michael, oxa-Michael, phospha-Michael, vinylogous-Michael, tandem-Michael etc. have been developed and well exploited for their synthetic applications. The reaction involving a nitrogen-based nucleophile as the Michael donor is known as the aza-Michael reaction, a thio-based as thio-Michael etc..
Enantioselective Michael Addition
For instance the asymmetric aza-Michael reaction alone or in tandem with other organic reaction(s) is an important synthetic tool to form new C–N bond(s) leading to develop new libraries of diverse types of biological active nitrogen compounds. Especially Cinchona alkaloids such as Cupreidine or Cupreine itself or squaramides, chiral bifunctional thioureas and phase-transfer catalysts based on the Cinchona framework have been widely used in asymmetric synthesis activating substrates through hydrogen bond formation. Most of these reactions are accompanied by high yields and extraordinary high enantiomeric excesses.
Lots of examples can be reviewed in the free of charge Chiral Catalyst Data Base. Thanks to this newly created Buchler Data Base, it is now possible to search specifically for individual problem solutions. Please contact us if you will require any special catalyst, which ist not part of our product portfolio.
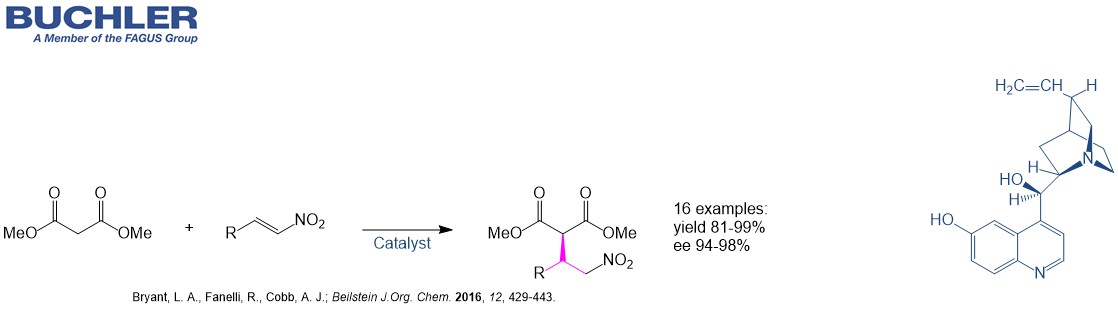
Example from Literature
Cupreines and cupreidines: an established class of bifunctional cinchona organocatalysts. (Bryant et al.; Beilstein J.Org. Chem. 2016, 12, 429-443.)
Further Articles:
(DHQ)2PHAL (CAS-No. 140924-50-1)
(DHQ)2PHAL - Dihydroquinine 1,4-phthalazinediyl diether (CAS-No. 140924-50-1) The well-known Cinchona alkaloid...
(DHQD)2PHAL (CAS-No. 140853-10-7)
(DHQD)2PHAL - Dihydroquinidine 1,4-phthalazinediyl diether (CAS-No. 140853-10-7) The well-known Cinchona alkaloid...
1,2-Addition
1,2-Addition is a type of organic chemical reaction that involves the addition of functional groups to the 1st and 2nd...
1,4-Addition (conjugate addition)
The Michael reaction is the conjugate 1,4-Addition of a resonance stabilized carbanion (michael donor) to an activated...
6`-Aminocinchonine (CAS-No. 2143936-31-4)
6´-Aminocinchonine Base (CAS-No. 2143936-31-4), a pseudoenantiomer of 6´-Aminocinchonidine, has the same...
